STUDYPRO Features
Click on any image to see a full-size preview of the user interface.
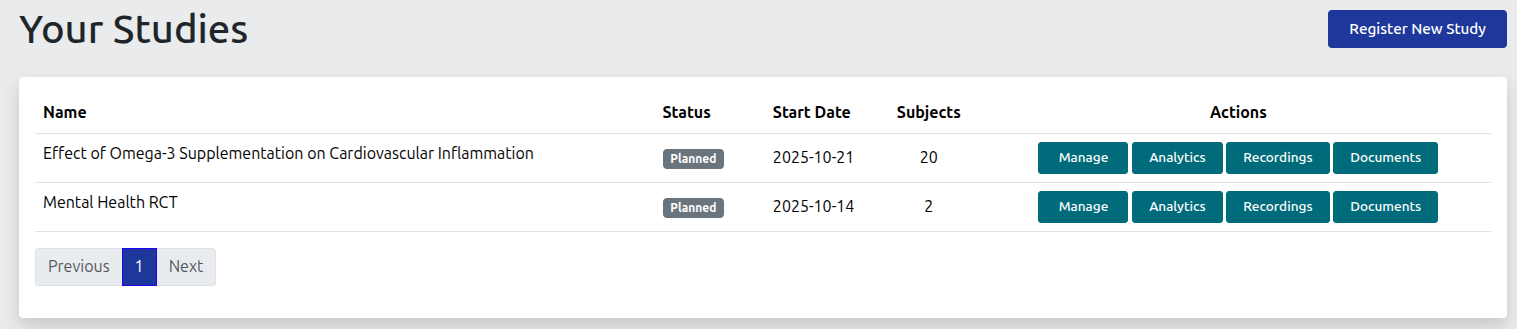
Central Dashboard
The central dashboard provides quick access to all studies a researcher has access to.
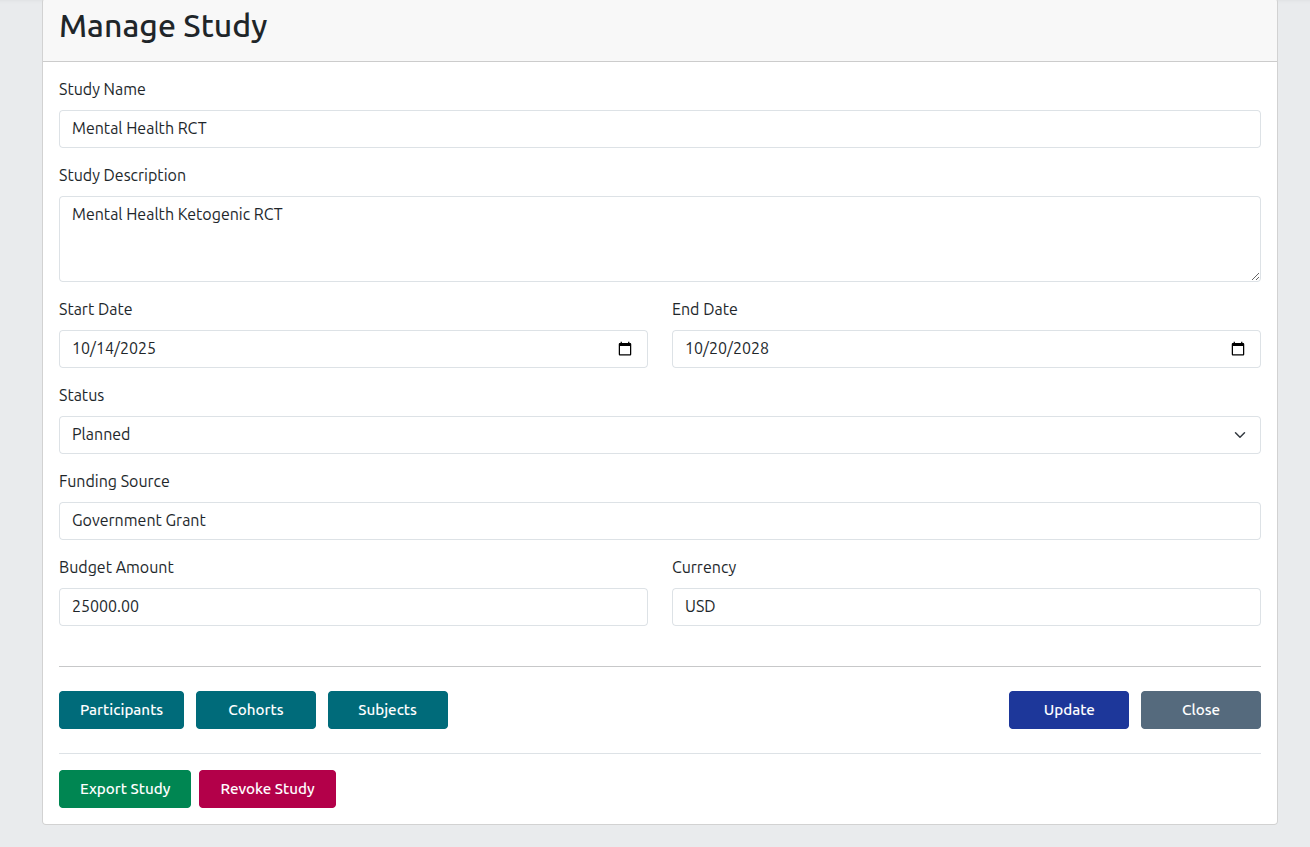
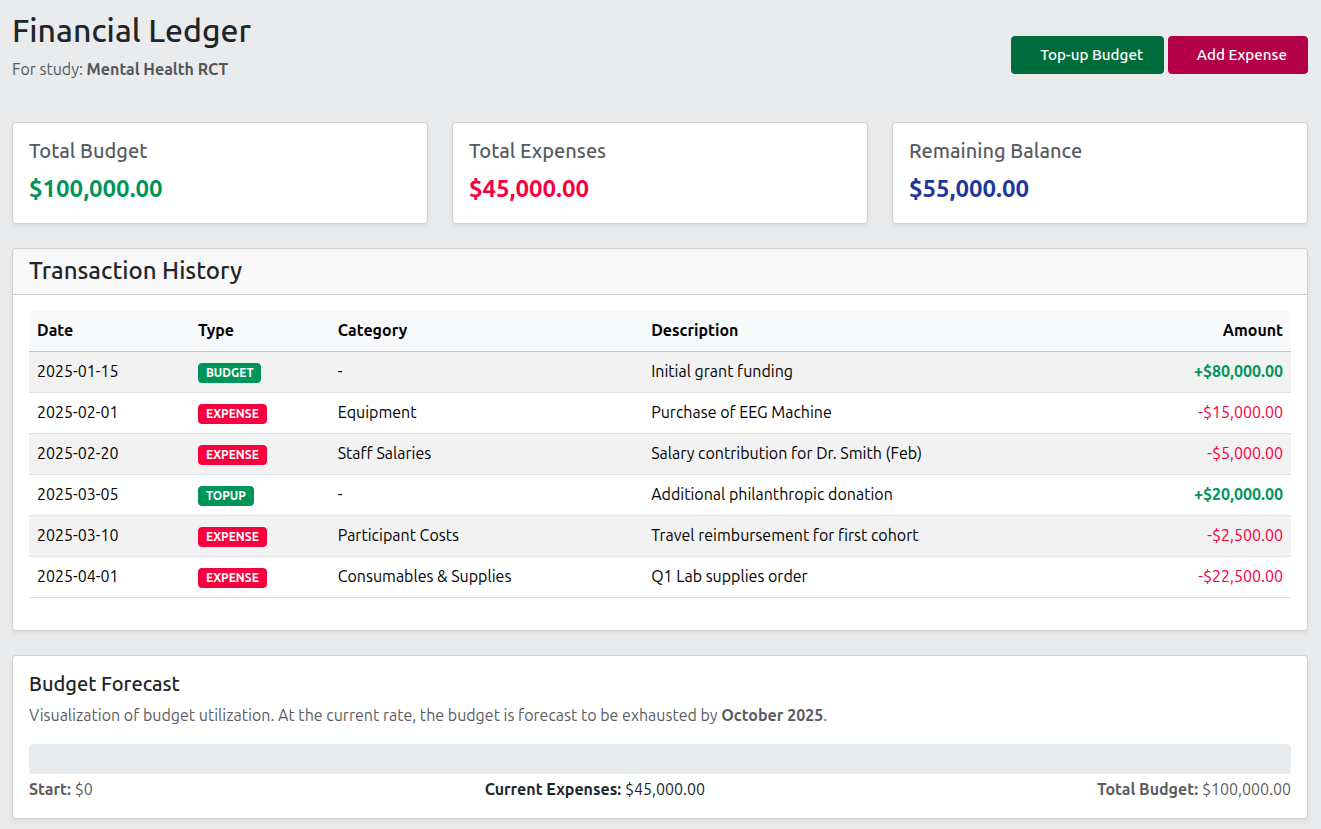
Study Registration
Quickly register new studies and track budget and grant allocation, or attach important documents such as ethics approvals.
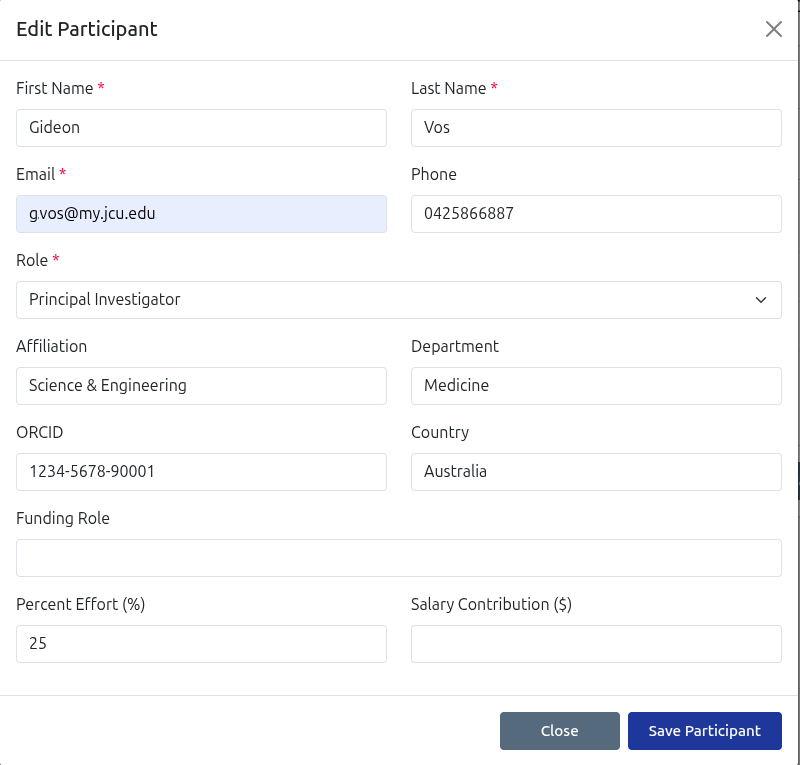
Participant Registration
Register any number of study participants with their research role and time allocation.
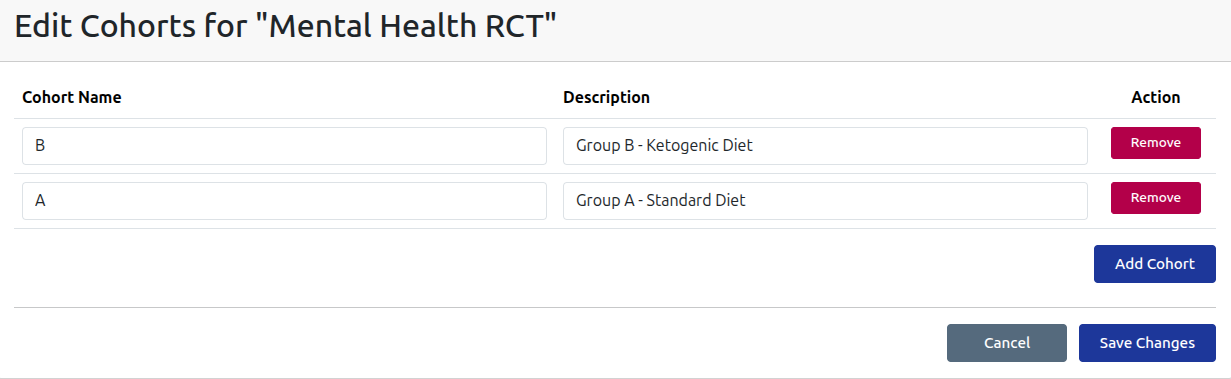
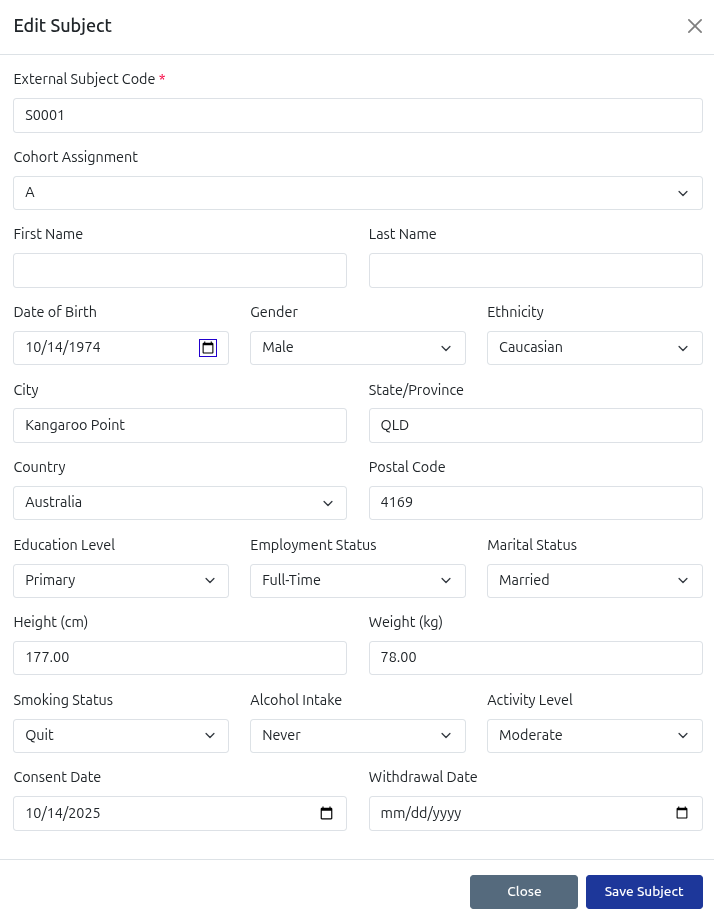
Subject Registration
Register trial subjects and allocate to cohort groups, with full support for medical history, health status and demographics. All details are optional to ensure subject privacy.
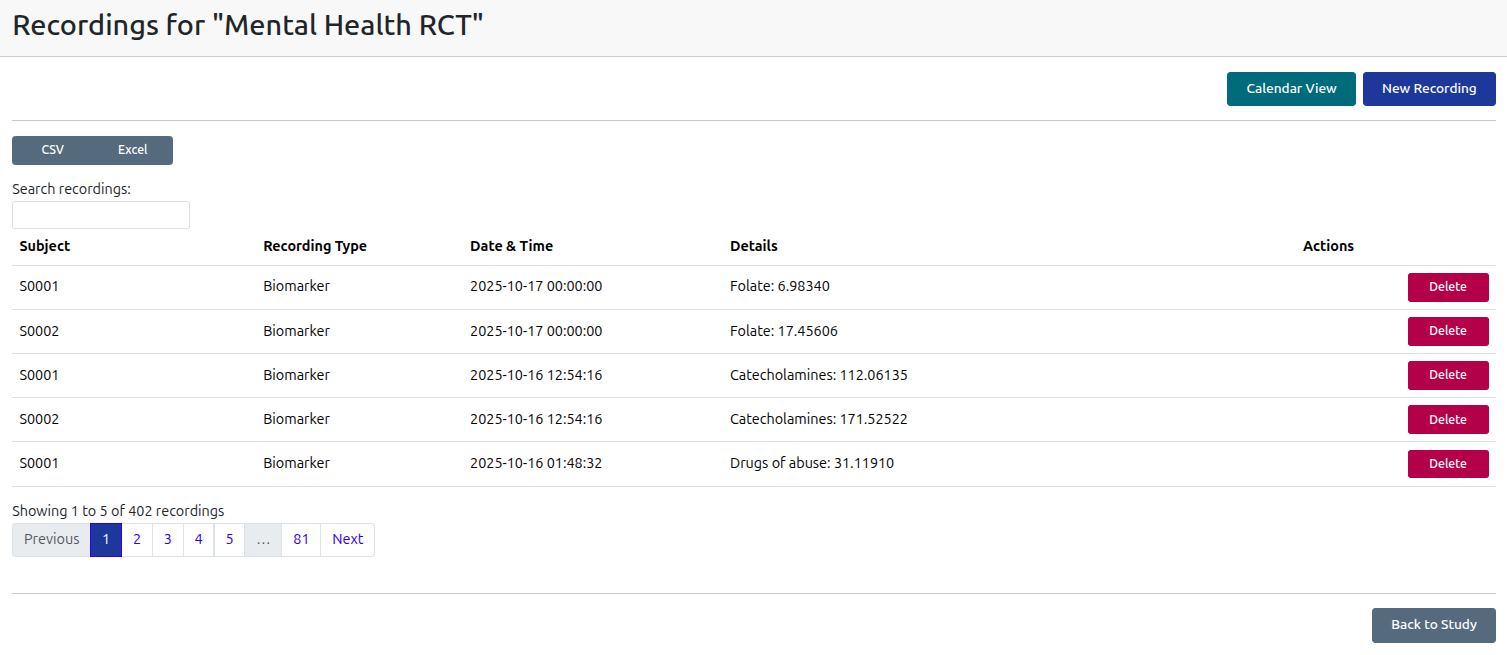
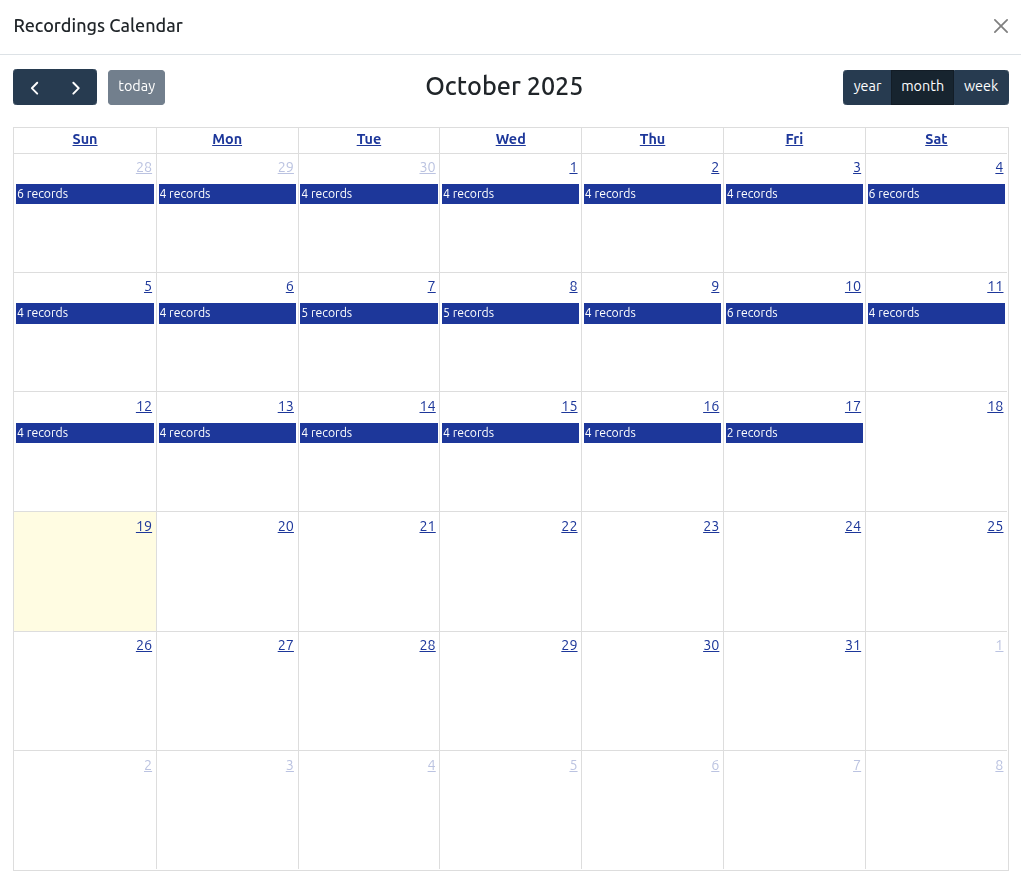
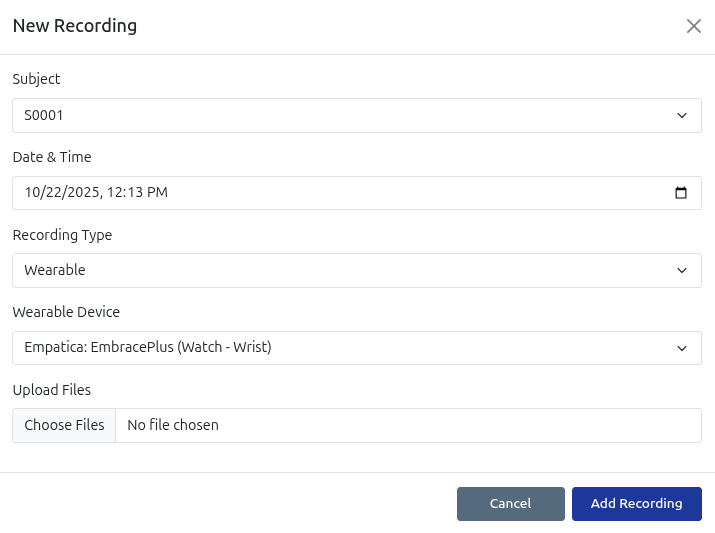
Biological and Digital Biomarker Recording
Record biological and digital biomarkers for tracking across a substantial range of biomarkers, wearable and EEG devices.
Device Data Archival
Upload and archive large wearable and EEG device datasets at low cost for any period of time, per subject.
Extensive Documentation Support
Assign and archive any number of documents per study and subject to track ethics approvals, consent and other key subject detail.
Built-In Analytics
We provide a range of visualizations and statistical tests that can be performed during trials.
Automated Reporting with Citations
Secure, privacy-enhanced A.I. analysis and insights generation with citation generation.