Why Adopt a Clinical Trial Management System (CTMS)?
Modern clinical research requires coordination across multiple disciplines, sites, and datasets. Spreadsheets, shared folders, and email-based workflows often result in fragmented information, poor audit trails, and data integrity risks. A dedicated CTMS centralizes every aspect of your study, from participant registration and biomarker tracking to analytics and compliance reporting.
By adopting a CTMS, research teams can:
- Ensure data consistency and integrity across every stage of a study.
- Automate regulatory compliance by maintaining complete audit logs and consent histories.
- Enhance team collaboration across departments and external partners with shared dashboards.
- Reduce administrative overhead by streamlining document management and financial tracking.
- Enable advanced analytics for faster insights and better decision-making during and after trials.
A dedicated Clinical Trial Management System eliminates manual errors, ensures traceability, and empowers teams to focus on science rather than administration.
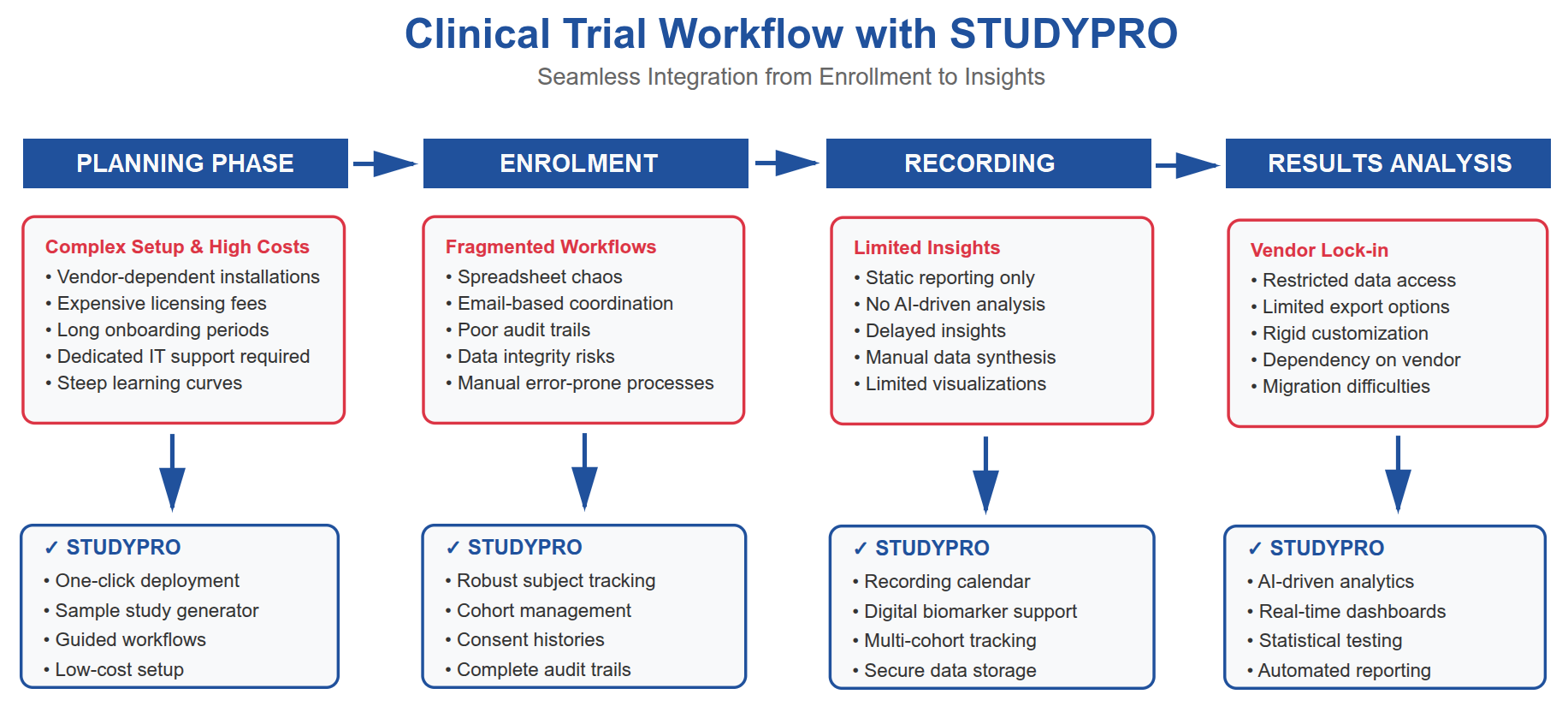
How We Solve Challenges You May Face in Your Study
STUDYPRO was designed by researchers, for researchers. It directly addresses the most common pain points encountered in academic, hospital-based, and multi-site studies without the complexity and cost of legacy CTMS platforms.
-
Challenge: Complex and expensive system setup.
Our solution: Deploy in minutes, either on-premises or cloud-hosted. No external consultants or multi-week installations required. -
Challenge: Steep learning curve for coordinators and students.
Our solution: An intuitive interface with guided study creation, built-in help content, and a Sample Study Generator for rapid onboarding and training. -
Challenge: Fragmented workflows across study types and data sources.
Our solution: Unified modules for participant management, cohort setup, biomarker and event recording with in-built statistical analysis, ensuring seamless data flow from enrollment to export. -
Challenge: Limited access to AI-driven insights in smaller trials.
Our solution: Integrated AI modules automatically summarize biomarker data, generate citations, and support early interpretation of trial outcomes. -
Challenge: High license and maintenance fees in commercial CTMS platforms.
Our solution: STUDYPRO is low-cost, lightweight, and scalable. It's designed to be affordable for academic institutions, smaller sponsors, and public research programs.
Where most CTMS platforms focus on enterprise clients with complex integration and regulatory compliance requirements, STUDYPRO focuses on accessibility, transparency, and usability. Every feature is built to simplify, not complicate, your trial operations.
How We Compare to Traditional CTMS Platforms
Traditional CTMS platforms are powerful but often expensive, and difficult to deploy, customize and maintain. They require long onboarding periods, dedicated IT support, and costly licensing models that put them out of reach for many research institutions.
| Feature | Traditional CTMS | STUDYPRO |
|---|---|---|
| Deployment | Requires vendor installation, long setup cycles | Ready-to-run with one-click cloud or local deployment |
| User Onboarding | Formal training sessions, steep learning curve | Guided workflows and sample study generator for rapid learning |
| Cost | High per-user or per-study licensing fees | Affordable academic and institutional pricing, low total cost of ownership |
| Customization | Rigid structure, vendor-dependent changes | Fully customizable as an open-source platform |
| Analytics | Limited to static reports | Dynamic dashboards, in-built statistics and AI-powered analytics |
| Compliance & Audit | Manual or third-party audit logs | Built-in audit trail with automatic compliance tracking |
| Support | Vendor-dependent, often costly | Direct support with transparent pricing |
Ready to Get Started?
Contact us to learn more about pricing and deployment options for your institution.